[ad_1]
A meta-analysis of two randomized studies evaluating patent foramen ovale (PFO) closure as a treatment strategy for migraine has shown significant benefits in several key endpoints, prompting the authors to conclude the approach warrants re-evaluation.
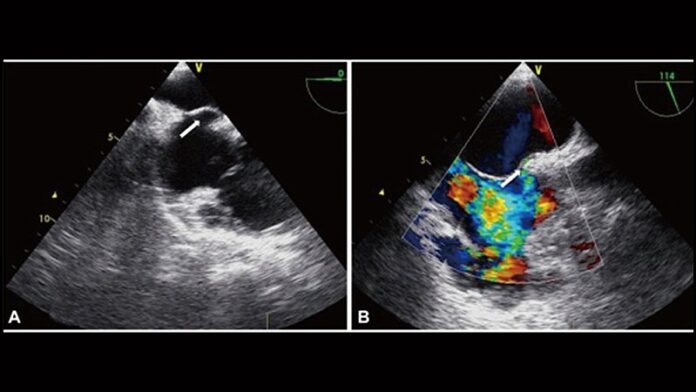
The pooled analysis of patient-level data from the PRIMA and PREMIUM studies, both of which evaluated the Amplatzer PFO Occluder device (Abbott Vascular), showed that PFO closure significantly reduced the mean number of monthly migraine days and monthly migraine attacks and resulted in a greater number of patients who experienced complete migraine cessation.
The study, led by Mohammad K. Mojadidi, MD, Virginia Commonwealth University, Richmond, was published online in the Journal of the American College of Cardiology on February 8.
Commenting for Medscape Medical News, coauthor of an accompanying editorial, Zubair Ahmed, MD, Cleveland Clinic, Ohio, said the meta-analysis gave some useful new information but is not enough to recommend PFO closure routinely for patients with migraine.
“This meta-analysis looked at different endpoints that are more relevant to current clinical practice than those in the two original studies, and the results show that we shouldn’t rule out PFO closure as a treatment strategy for some migraine patients,” Ahmed stated. “But we’re still not sure exactly which patients are most likely to benefit from this approach, and we need additional studies to gain more understanding on that.”
In the JACC article, the study authors note that there is an established link between the presence of PFO and migraine, especially migraine with aura. In observational studies of PFO closure for cryptogenic stroke, the vast majority of patients who also had migraine reported a more than 50% reduction in migraine days per month after PFO closure.
However, two recent randomized clinical trials evaluating the Amplatzer PFO Occluder device for reducing the frequency and duration of episodic migraine headaches did not meet their respective primary endpoints, although they did show significant benefit of PFO closure in most of their secondary endpoints.
The current meta-analysis pooled individual participant data from the two trials to increase the power to detect the effect of percutaneous PFO closure for treating patients with episodic migraine compared with medical therapy alone.
In the two studies including a total of 337 patients, 176 were randomized to PFO closure and 161 to medical treatment only. At 12 months, three of the four efficacy endpoints evaluated in the meta-analysis were significantly reduced in the PFO closure group. These were mean reduction of monthly migraine days (–3.1 days vs –1.9 days; P = .02), mean reduction of monthly migraine attacks (–2.0 vs –1.4; P = .01), and number of patients who experienced complete cessation of migraine (9% vs 0.7%; P < .001).
The responder rate, defined as more than a 50% reduction in migraine attacks, showed a trend towards an increase in the PFO closure group but did not achieve statistical significance (38% vs 29%; P = .13).
For the safety analysis, nine procedure-related and four device-related adverse events occurred in 245 patients who eventually received devices. All events were transient and resolved.
Better Effect in Patients With Aura
Patients with migraine with aura, in particular frequent aura, had a significantly greater reduction in migraine days and a higher incidence of complete migraine cessation following PFO closure versus no closure, the authors report.
In those without aura, PFO closure did not significantly reduce migraine days or improve complete headache cessation. However, some patients without aura did respond to PFO closure, which was statistically significant for reduction of migraine attacks (–2.0 vs –1.0; P = .03).
“The interaction between the brain that is susceptible to migraine and the plethora of potential triggers is complex. A PFO may be the potential pathway for a variety of chemical triggers, such as serotonin from platelets, and although less frequent, some people with migraine without aura may trigger their migraine through this mechanism,” the researchers suggest. This hypothesis will be tested the RELIEF trial now being planned.
In the accompanying editorial, Ahmed and coauthor Robert J. Sommer, MD, Columbia University Medical Center, New York City, point out that the meta-analysis demonstrates benefit of PFO closure in the migraine population for the first time.
“Moreover, the investigators defined a population of patients who may benefit most from PFO closure, those with migraine with frequent aura, suggesting that these may be different physiologically than other migraine subtypes. The analysis also places the PRIMA and PREMIUM outcomes in the context of endpoints that are more practical and are more commonly assessed in current clinical trials,” they note.
But the editorialists highlight several significant limitations of the analysis including “pooling of patient cohorts, methods, and outcome measures that might not be entirely comparable,” which they say could have introduced bias.
They also point out that the underlying pathophysiologic mechanism linking migraine symptoms to PFO remains unknown. They explain that the mechanism is thought to involve the right-to-left passage of systemic venous blood, with some component — which would normally be eliminated or reduced on passage through the pulmonary vasculature — reaching the cerebral circulation via the PFO in supranormal concentrations and acting as a trigger for migraine activity in patients with susceptible brains.
But not all patients with migraine who have PFO benefit from PFO closure, they note, and therefore presumably have PFO-unrelated migraines. There is no verified way to distinguish between these two groups at present.
“Once we learn to identify the subset of migraine patients in whom PFOs are actually causal of headache symptoms, screening and treatment of PFO for migraine can become a reality,” they write.
Although the meta-analysis is a step in the right direction, “it is not a home run,” Ahmed elaborated. “This was a post-hoc analysis of two studies, neither of which showed significant benefits on their primary endpoints. That weakens the findings somewhat.”
He added: “At present, PFO closure is not routinely recommended as a migraine treatment strategy as we haven’t been sure which patients are most likely to benefit. And while this meta-analysis suggests patients with aura may be more likely to benefit, one quarter of patients without aura in the PREMIUM trial responded to PFO closure, so it’s not just about aura.”
“There are still many unanswered questions.”
“I don’t think the new information from this meta-analysis is enough to persuade me to change my practice, but it is a small building block in the overall picture and suggests this may be a suitable strategy for some patients in future,” he concluded.
The study had no outside funding. Participant-level data were provided by Abbott. Several coauthors were on the steering committee for the PREMIUM or PRIMA trials. Ahmed has reported receiving consulting fees from Eli Lilly, Amgen, AbbVie, and electroCore; serving on advisory boards for Amgen and Supernus; serving as a speaker for AbbVie; and receiving funding for an investigator-initiated trial from Teva and Eli Lilly.
J Am Coll Cardiol. Published online February 8, 2021. Abstract, Editorial
For more Medscape Neurology news, join us on Facebook and Twitter.
[ad_2]
Source link