[ad_1]
Summary: A new vestibular implant can significantly reduce dizziness, restore balance, and improve the quality of life for people with bilateral vestibular hypofunction (BVH).
Source: Johns Hopkins Medicine
Getting around without the need to concentrate on every step is something most of us can take for granted because our inner ears drive reflexes that make maintaining balance automatic. However, for about 1.8 million adults worldwide with bilateral vestibular hypofunction (BVH) — loss of the inner ears’ sense of balance — walking requires constant attention to avoid a fall.
Now, Johns Hopkins Medicine researchers have shown that they can facilitate walking, relieve dizziness and improve quality of life in patients with BVH by surgically implanting a stimulator that electrically bypasses malfunctioning areas of the inner ear and partially restores the sensation of balance.
Results from their study of eight patients using the device are published today in the New England Journal of Medicine.
To maintain balance while moving through the world around us, our brains receive and process data from multiple sensory systems, including vision, proprioception (muscles and joints) and vestibular sensation from the inner ears. People with BVH have difficulty keeping their eyes, head and body steady. Head movements make their vision jump and blur, and walking requires conscious effort. Forced to deal with this mental distraction, individuals with BVH suffer a more than thirtyfold increase in fall risk and the social stigma of appearing to walk like someone who’s intoxicated.
Current therapy for BVH is limited to vestibular rehabilitation exercises. Doctors advise their patients with BVH to avoid medications that damage the inner ear (ototoxic drugs) or suppress brain function (sedatives), and caution them to steer clear of activities that might endanger them or others, such as driving, swimming and walking in poorly lit areas.
“Although about 20 individuals had been implanted elsewhere with devices used to stimulate the vestibular nerve in a laboratory setting, participants in this trial are true pioneers — the first to use a vestibular implant as a long-term, 24-hour-per-day sensory restoration treatment,” says senior study author Charley Della Santina, M.D., Ph.D., professor of otolaryngology-head and neck surgery and biomedical engineering at the Johns Hopkins University School of Medicine and director of the Johns Hopkins Vestibular NeuroEngineering Laboratory, which conducted the study.
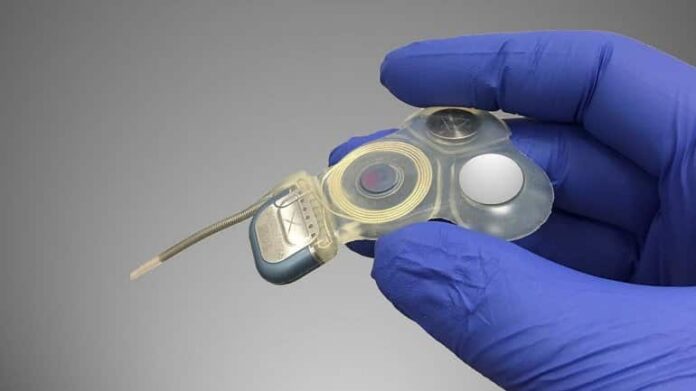
To achieve this milestone, Della Santina and his colleagues used basic research and engineering technology to modify a cochlear implant — a device that improves hearing loss by electrically stimulating the inner ear’s cochlear nerve — to instead activate the nearby vestibular nerve in response to signals from a motion sensor on the patient’s head.
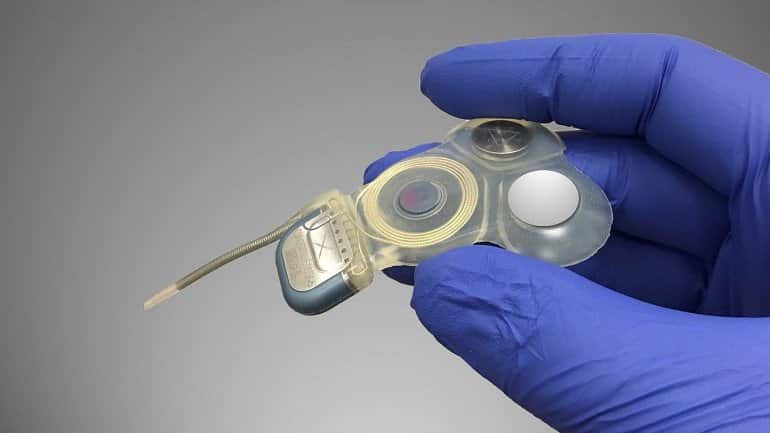
Electrical pulse strength and timing convey information about the speed and direction of the patient’s head motion which, in turn, drives head and eye reflexes that help maintain clearer vision during head movement and reduce the need to exert conscious effort to avoid falls.
In their study, the Johns Hopkins Medicine researchers evaluated eight patients with BVH who received the vestibular implant, assessing changes in postural stability, walking, hearing and patient-reported outcomes, including dizziness and quality of life.
Assessments were conducted before implantation surgery (the baseline measure) and at six months and one year afterward. Median scores improved for the group on four of the five posture and gait metrics, and on three of the four patient-reported outcomes.
All eight patients experienced some hearing loss in the implanted ear. Five maintained hearing in the implanted ear sufficient to use a telephone without a hearing aid, and three experienced greater hearing loss.
“Improvement in performance on standardized clinical tests of balance and walking has been remarkable,” says Margaret Chow, study lead author and biomedical engineering doctoral candidate at The Johns Hopkins University. “Even more gratifying is that our patients have been able to return to activities that enrich their daily lives, such as exercising, riding a bike, gardening or dancing at a daughter’s wedding.”
Overall, the improvement in quality of life and relief from the misery of BVH has been life altering, says A’ndrea Messer, Ph.D., one of the patients chronicled in the Johns Hopkins Medicine study and a senior science and research information officer at Penn State University.
“The multichannel vestibular implant is incredible,” says Messer. “Before receiving it, I couldn’t walk in the dark, on uneven ground or without a cane. Now, I can do all of those things and am living a fairly normal life.”
Along with Della Santina and Chow, the research team members from the Johns Hopkins University School of Medicine are Andrianna Ayiotis, Peter Boutros, Stephen Bowditch, John Carey, Yoav Gimmon, Carolina Treviño Guajardo, Kelly Lane, Brian Morris, Desi Schoo, Michael Schubert, Daniel Sun and Bryan Ward. Team members from industry sponsor Labyrinth Devices LLC are engineers Mehdi Rahman and Nicolas Valentin, both alumni of the Vestibular NeuroEngineering Laboratory.
Funding: The study was supported by grants R01DC013536 and 2T32DC000023 from the National Institute on Deafness and Other Communications Disorders.
The Johns Hopkins University and Labyrinth Devices LLC, of which Della Santina is founder and CEO, hold royalty interests in pending and awarded patents for the vestibular implant used in this study. Terms of this agreement are managed in accordance with university policies on conflict of interest.
About this auditory neuroscience and neuroengineering research news
Source: Johns Hopkins University
Contact: Michael E. Newman – Johns Hopkins University
Image: The image is credited to Johns Hopkins Vestibular NeuroEngineering Laboratory
Original Research: Closed access.
“Posture, Gait, Quality of Life, and Hearing with a Vestibular Implant” by Charley Della Santina et al. NEJM
Abstract
Posture, Gait, Quality of Life, and Hearing with a Vestibular Implant
Table of Contents
BACKGROUND
Bilateral vestibular hypofunction is associated with chronic disequilibrium, postural instability, and unsteady gait owing to failure of vestibular reflexes that stabilize the eyes, head, and body. A vestibular implant may be effective in alleviating symptoms.
METHODS
Persons who had had ototoxic (7 participants) or idiopathic (1 participant) bilateral vestibular hypofunction for 2 to 23 years underwent unilateral implantation of a prosthesis that electrically stimulates the three semicircular canal branches of the vestibular nerve. Clinical outcomes included the score on the Bruininks–Oseretsky Test of Motor Proficiency balance subtest (range, 0 to 36, with higher scores indicating better balance), time to failure on the modified Romberg test (range, 0 to 30 seconds), score on the Dynamic Gait Index (range, 0 to 24, with higher scores indicating better gait performance), time needed to complete the Timed Up and Go test, gait speed, pure-tone auditory detection thresholds, speech discrimination scores, and quality of life. We compared participants’ results at baseline (before implantation) with those at 6 months (8 participants) and at 1 year (6 participants) with the device set in its usual treatment mode (varying stimulus pulse rate and amplitude to represent rotational head motion) and in a placebo mode (holding pulse rate and amplitude constant).
RESULTS
The median scores at baseline and at 6 months on the Bruininks–Oseretsky test were 17.5 and 21.0, respectively (median within-participant difference, 5.5 points; 95% confidence interval [CI], 0 to 10.0); the median times on the modified Romberg test were 3.6 seconds and 8.3 seconds (difference, 5.1; 95% CI, 1.5 to 27.6); the median scores on the Dynamic Gait Index were 12.5 and 22.5 (difference, 10.5 points; 95% CI, 1.5 to 12.0); the median times on the Timed Up and Go test were 11.0 seconds and 8.7 seconds (difference, 2.3; 95% CI, −1.7 to 5.0); and the median speeds on the gait-speed test were 1.03 m per second and 1.10 m per second (difference, 0.13; 95% CI, −0.25 to 0.30). Placebo-mode testing confirmed that improvements were due to treatment-mode stimulation. Among the 6 participants who were also assessed at 1 year, the median within-participant changes from baseline to 1 year were generally consistent with results at 6 months. Implantation caused ipsilateral hearing loss, with the air-conducted pure-tone average detection threshold at 6 months increasing by 3 to 16 dB in 5 participants and by 74 to 104 dB in 3 participants. Changes in participant-reported disability and quality of life paralleled changes in posture and gait.
CONCLUSIONS
Six months and 1 year after unilateral implantation of a vestibular prosthesis for bilateral vestibular hypofunction, measures of posture, gait, and quality of life were generally in the direction of improvement from baseline, but hearing was reduced in the ear with the implant in all but 1 participant. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT02725463. opens in new tab.)
[ad_2]
Source link