[ad_1]
Summary: Cells that drive myelin repair become less efficient due to aging. Myelin loss results in cognitive decline and is central to a number of neurodegenerative diseases.
Source: University of Portsmouth
A new study led by the University of Portsmouth has identified that one of the major factors of age-related brain deterioration is the loss of a substance called myelin.
Myelin acts like the protective and insulating plastic casing around the electrical wires of the brain – called axons. Myelin is essential for superfast communication between nerve cells that lie behind the supercomputer power of the human brain.
The loss of myelin results in cognitive decline and is central to several neurodegenerative diseases, such as Multiple Sclerosis and Alzheimer’s disease. This new study found that the cells that drive myelin repair become less efficient as we age and identified a key gene that is most affected by ageing, which reduces the cells ability to replace lost myelin.
The study, published this week in the journal Ageing Cell, is part of an international collaboration led by Professor Arthur Butt at the University of Portsmouth with Dr Kasum Azim at the University of Dusseldorf in Germany, together with Italian research groups of Professor Maria Pia Abbracchio in Milan and Dr Andrea Rivera in Padua.
Professor Butt said: “Everyone is familiar with the brain’s grey matter, but very few know about the white matter, which comprises of the insulated electrical wires that connect all the different parts of our brains.
“A key feature of the ageing brain is the progressive loss of white matter and myelin, but the reasons behind these processes are largely unknown. The brain cells that produce myelin – called oligodendrocytes – need to be replaced throughout life by stem cells called oligodendrocyte precursors. If this fails, then there is a loss of myelin and white matter, resulting in devastating effects on brain function and cognitive decline. An exciting new finding of our study is that we have uncovered one of the reasons that this process is slowed down in the aging brain.”
Dr Rivera, lead author of the study while he was in University of Portsmouth and who is now a Fellow at the University of Padua, explained: “By comparing the genome of a young mouse brain to that of a senile mouse, we identified which processes are affected by ageing. These very sophisticated analysis allowed us to unravel the reasons why the replenishment of oligodendrocytes and the myelin they produce is reduced in the aging brain.
“We identified GPR17, the gene associated to these specific precursors, as the most affected gene in the ageing brain and that the loss of GPR17 is associated to a reduced ability of these precursors to actively work to replace the lost myelin.”
The work is still very much ongoing and has paved the way for new studies on how to induce the ‘rejuvenation’ of oligodendrocyte precursor cells to efficiently replenish lost white matter.
Dr Azim of the University of Dusseldorf said: “This approach is promising for targeting myelin loss in the aging brain and demyelination diseases, including Multiple Sclerosis, Alzheimer’s disease and neuropsychiatric disorders. Indeed, we have only touched the tip of the iceberg and future investigation from our research groups aim to bring our findings into human translational settings.”
Dr Rivera performed the key experiments published in this study while at the University of Portsmouth and he has been awarded the prestigious MSCA Seal of Excellence @UniPD Fellowship to translate these findings and investigate this further in the human brain, in collaboration with Professors Raffele De Caro, Andrea Porzionato and Veronica Macchi at the Institute of Human Anatomy of the University of Padua.
The study was funded by grants from the BBSRC and MRC to Professor Butt, together with the UK and Italian MS Societies (to Professors Butt and Abbracchio, respectively), and the Swiss National Funds Fellowship and German Research Council (Dr Azim). Dr Andrea Rivera was supported by an Anatomical Society PhD Studentship (with Professor Butt), and the MSCA Seal of Excellence @UniPD (Dr Rivera).
Dr Emma Gray, Assistant Director of Research at the MS Society, said: “MS can be relentless and painful, and there are sadly still no treatments to stop disability progression. We can see a future where no one has to worry about MS getting worse but, for that to happen, we need to find ways to repair damaged myelin. This research sheds light on why cells that drive myelin repair become less efficient as we age, and we’re really proud to have helped fund it. By improving our understanding of ageing brain stem cells, it gives us a new target to help slow the progression of MS, and could have important implications for future treatment.”
About this aging and neurology research news
Source: University of Portsmouth
Contact: Press Office – University of Portsmouth
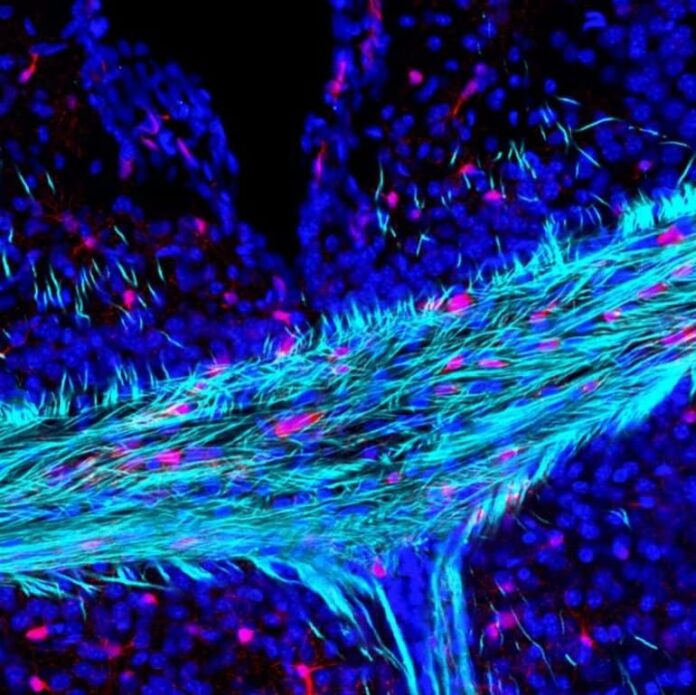
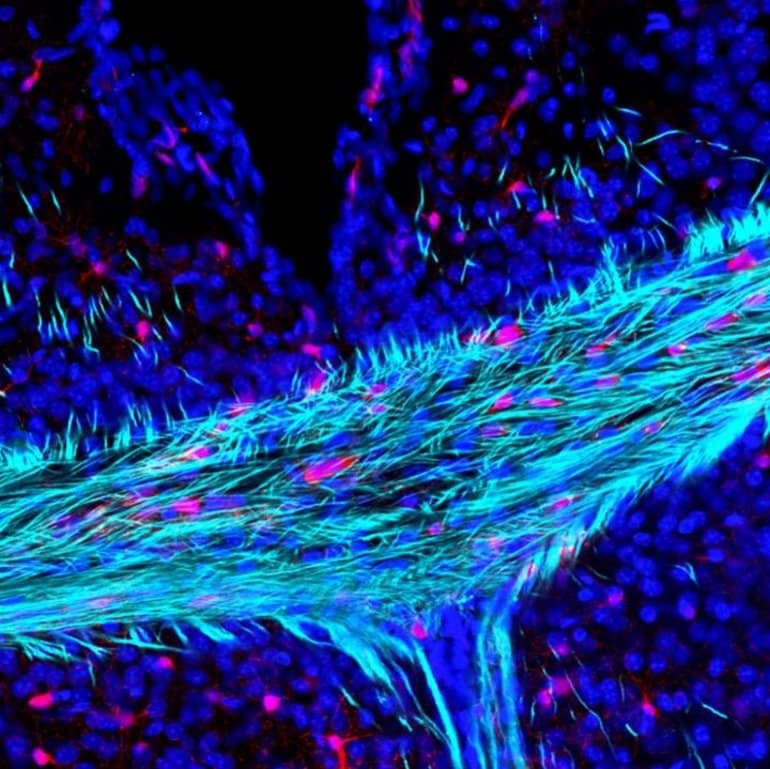
Image: The image is credited to Andrea Rivera
Original Research: Open access.
“Functional genomic analyses highlight a shift in Gpr17‐regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum” by Andrea Rivera et al. Aging Cell
Abstract
Functional genomic analyses highlight a shift in Gpr17‐regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum
Brain ageing is characterised by a decline in neuronal function and associated cognitive deficits. There is increasing evidence that myelin disruption is an important factor that contributes to the age‐related loss of brain plasticity and repair responses. In the brain, myelin is produced by oligodendrocytes, which are generated throughout life by oligodendrocyte progenitor cells (OPCs).
Currently, a leading hypothesis points to ageing as a major reason for the ultimate breakdown of remyelination in Multiple Sclerosis (MS). However, an incomplete understanding of the cellular and molecular processes underlying brain ageing hinders the development of regenerative strategies.
Here, our combined systems biology and neurobiological approach demonstrate that oligodendroglial and myelin genes are amongst the most altered in the ageing mouse cerebrum. This was underscored by the identification of causal links between signalling pathways and their downstream transcriptional networks that define oligodendroglial disruption in ageing.
The results highlighted that the G‐protein coupled receptor Gpr17 is central to the disruption of OPCs in ageing and this was confirmed by genetic fate‐mapping and cellular analyses.
Finally, we used systems biology strategies to identify therapeutic agents that rejuvenate OPCs and restore myelination in age‐related neuropathological contexts.
[ad_2]
Source link