[ad_1]
Summary: A new zebrafish model holds the potential for future studies of glioblastoma, an aggressive and lethal brain cancer.
Source: Virginia Tech
Scientists at the Fralin Biomedical Research Institute at VTC have identified a new zebrafish model that could help advance glioblastoma multiforme research. Glioblastoma is an aggressive form of primary brain tumor – fewer than one in 20 patients survive five years after diagnosis.
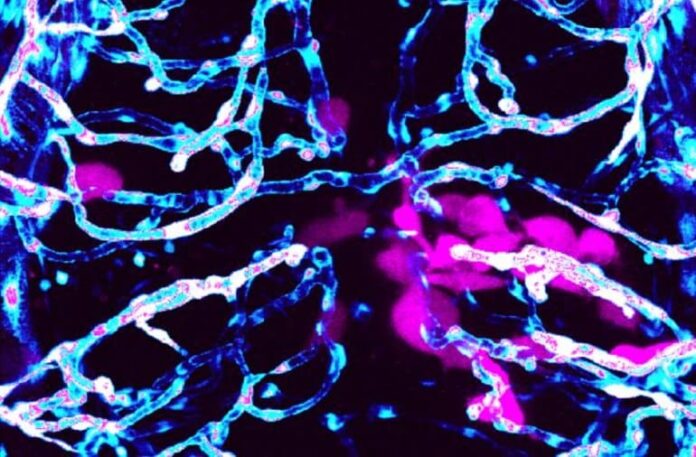
The research team previously discovered that human-derived brain cancer cells in mice use the brain’s blood vessels like highways to spread away from the original mass. In the new study, published in ACS Pharmacology and Translational Science, they show clear cross-over between mammals and fish and describe similar observations in zebrafish.
“Our hope is that this new work in zebrafish will help researchers efficiently evaluate new, much-needed therapeutics in a pre-clinical animal model to target these aggressive and devastating tumors,” said Harald Sontheimer, formerly a professor at the Fralin Biomedical Research Institute at the time of the study.
When brain cancer cells disperse, they become harder to locate and delete. Even when the original tumor is surgically removed, migratory glioblastoma cells can linger – undetected by diagnostic imaging – eventually forming new satellite tumors if they can endure chemotherapy or radiation therapies.
As the cancer cells migrate, they also leave a destructive wake.
“Our previous research showed that glioma cells exploit the brain’s blood vessels. Tumor cells use blood vessels as a pathway to invade within the brain and consequently break down the blood-brain barrier,” said Robyn Umans, postdoctoral associate in Sontheimer’s laboratory at the Fralin Biomedical Research Institute and the study’s lead author.
But what if there were a way to block glioma cells from leveraging blood vessels to control cancer cell migration?
Rodent studies led by other researchers revealed that gliomas need a key signaling pathway – Wnt, named for the wingless flies it was first observed in – in order to exploit blood vessels. When Umans added a Wnt inhibitor molecule to the zebrafish’s water, they noticed a change in glioma cell behavior — the cancer cells had reduced attachments to the brain’s vasculature.
Zebrafish skin and scales are crystal clear. Scientists can put the fish under a microscope and, with fluorescent markers, watch cancer cells grow, migrate, and interact with other cells – all in real-time.
Fast-growing zebrafish are also efficient, less expensive to maintain than other preclinical models, and they share 70 percent of the same DNA as humans.

“For me, seeing is believing. With zebrafish models I can see biological mechanisms underlying aggressive cancers come to life in real-time, gaining this unprecedented visibility into the tumor microenvironment,” Umans said. “Our hope is that these proof-of-principal experiments validate the zebrafish model as an additional platform for cancer drug discovery.”
The researchers also wanted to see if the glioma cells also metastasized using blood vessels outside of the brain, so they injected a handful of cells into the fish’s peripheral tissue. This group of cancer cells attempted to travel to the brain, but they didn’t latch onto any pre-existing blood vessels. Instead, nearby vessels appeared to stem offshoots that grew toward the cancer cells like a magnet, suggesting angiogenesis, the growth of new blood vessels – a hallmark of many cancers that helps assure steady nutrient supply.
“This was a rare and special opportunity to, as a postdoc, establish a new model organism for a lab,” Umans said. “This study was an exciting collaboration because two of the authors were undergraduate researchers who contributed significantly to imaging and analysis, as well as a senior principal investigator. It was really rewarding to mentor these amazing, young scientists and see a study go from the creation of a fish room to a publication.”
About this glioblastoma brain cancer research news
Source: Virginia Tech
Contact: Whitney Slightham – Virginia Tech
Image: The image is credited to Robyn Umans/Virginia Tech
Original Research: Closed access.
“Fishing for Contact: Modeling Perivascular Glioma Invasion in the Zebrafish Brain” by Harald Sontheimer et al. ACS Pharmacology & Translational Science
Abstract
Fishing for Contact: Modeling Perivascular Glioma Invasion in the Zebrafish Brain
Glioblastoma multiforme (GBM) is a highly invasive, central nervous system (CNS) cancer for which there is no cure. Invading tumor cells evade treatment, limiting the efficacy of the current standard of care regimen. Understanding the underlying invasive behaviors that support tumor growth may allow for generation of novel GBM therapies. Zebrafish (Danio rerio) are attractive for genetics and live imaging and have, in recent years, emerged as a model system suitable for cancer biology research. While other groups have studied CNS tumors using zebrafish, few have concentrated on the invasive behaviors supporting the development of these diseases. Previous studies demonstrated that one of the main mechanisms of GBM invasion is perivascular invasion, i.e., single tumor cell migration along blood vessels. Here, we characterize phenotypes, methodology, and potential therapeutic avenues for utilizing zebrafish to model perivascular GBM invasion. Using patient-derived xenolines or an adherent cell line, we demonstrate tumor expansion within the zebrafish brain. Within 24-h postintracranial injection, D54-MG-tdTomato glioma cells produce fingerlike projections along the zebrafish brain vasculature. As few as 25 GBM cells were sufficient to promote single cell vessel co-option. Of note, these tumor-vessel interactions are CNS specific and do not occur on pre-existing blood vessels when injected into the animal’s peripheral tissue. Tumor-vessel interactions increase over time and can be pharmacologically disrupted through inhibition of Wnt signaling. Therefore, zebrafish serve as a favorable model system to study perivascular glioma invasion, one of the deadly characteristics that make GBM so difficult to treat.
[ad_2]
Source link